You’re about to visit a third-party site that isn’t controlled by P1 Technologies and may have different privacy and security practices. Do you want to continue?
Millions of patients worldwide rely on medical devices every day—ranging from simple tools that monitor blood pressure to advanced systems that diagnose disease. Whether used externally or implanted within the body, these devices must be safe and dependable. To ensure this, regulatory agencies such as the U.S. Food and Drug Administration (FDA), the European Union (EU), and others around the world require that medical devices meet strict safety and compliance standards before they can be sold.
At P1 Technologies, we design our products to meet the highest standards of safety, health, and environmental compliance worldwide. We are committed to providing high-quality products that conform to global regulations, including REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) and RoHS (Restriction of Hazardous Substances). Our team continuously monitors evolving regulations to ensure that our products remain fully compliant, because we understand how critical this is for our customers and the patients they serve.
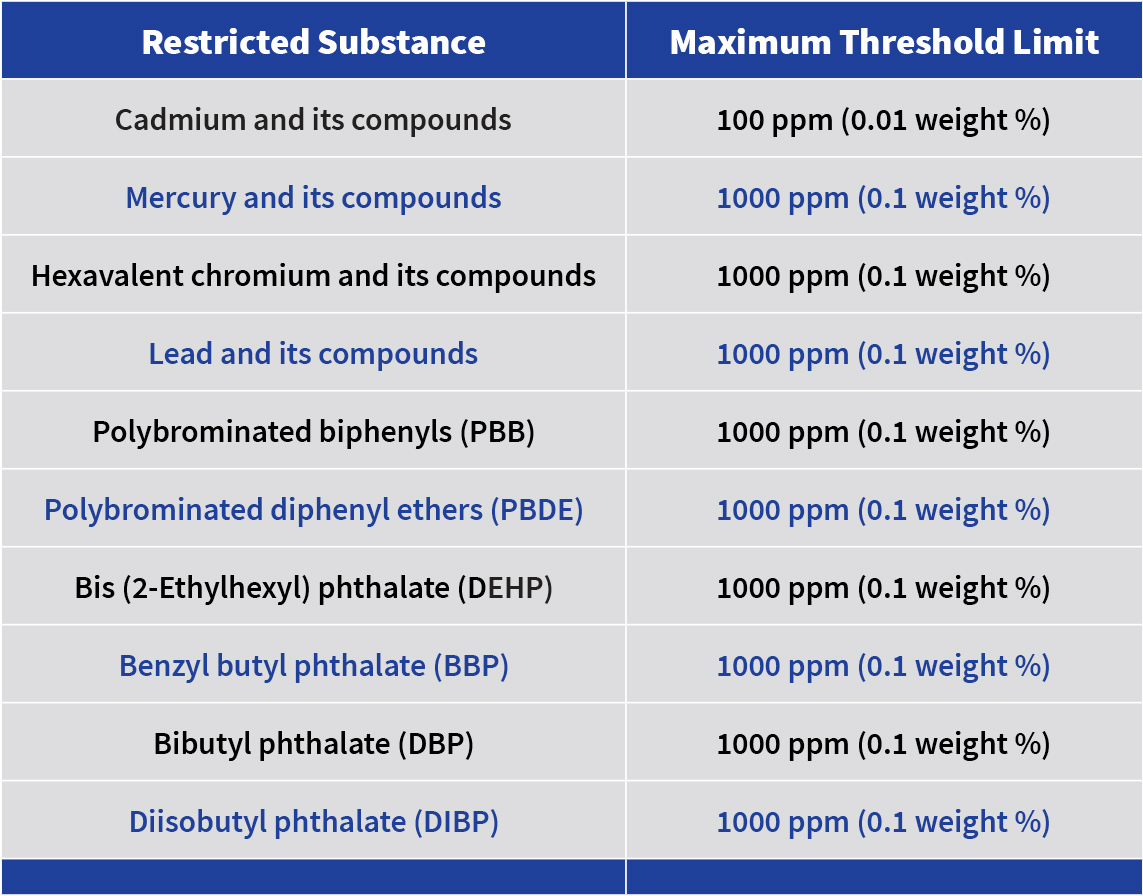
P1 Technologies has been producing parts that are RoHS compliant for many years. The majority of our products are RoHS compliant. These products are indicated as compliant on our packing slips. RoHS compliant means that substances restricted by EU Directive 2002/95/EC and subsequent amendments of the European Parliament are not contained in a finished product above threshold limits published by the European Commission excluding exempted applications.
Unfortunately, P1 Technologies cannot guarantee that every product that is made can comply with RoHS. However, the majority of our parts are compliant to RoHS 2015/865.
REACH establishes procedures for collecting and assessing information on the properties and hazards of substances with the purpose of providing a high level of protection of human health and the environment from the use of chemicals.
As with RoHS, we cannot guarantee that every product that is made can comply with REACH. P1 Technologies works tirelessly to meet our customer’s specifications and requirements regarding their products.
P1 Technologies recognizes and supports the Dodd-Frank Wall Street Reform and Consumer Protection Act, Section 1502 concerning Conflict Minerals. As a privately held company we are not required to report to the Security and Exchange Commission; however, we do accept our responsibility to support our customers and human rights.
Our goal is to identify and eliminate from our manufacturing processes any minerals sourced from the Democratic Republic of Congo or neighboring countries unless verified by the Responsible Minerals Initiative. P1 Technologies practices reasonable due diligence by surveying and partnering with suppliers that share our goal.
P1 Technologies provides a declaration of whether “natural rubber” [including natural rubber latex, dry natural rubber, and synthetic latex or rubber that includes natural rubber in its formulation] is intentionally added as an ingredient to the raw materials used in the finished good listed below. P1 does not knowingly add “natural rubber” in the production of finished goods. Following FDA guidance document 1768, P1 will not state product or packaging are “latex free” or “do not contain latex”.
Animal-Derived Materials
The outer jacket for our wires contains Bovine Tallow Stearate. Stearates bind to and inactivate trace polymerization catalysts remaining in polymer blends. Our supplier has assured us that their material complies with the following:
Based on the above information, we are confident that our product presents minimal risk for the introduction and spread of infectious prion proteins that can lead to transmissible spongiform encephalopathies (TSE).
For raw materials used in the production of medical grade components, P1 maintains a database of available statements and/or technical data obtained from manufacturers regarding biocompatibility. For more information regarding biocompatibility specifics, please contact your sales representative.
Please note that P1 Technologies, Inc. does not perform chemical analysis or testing of materials supplied to us. This declaration provides information on the above listed part numbers using the current lot of materials. This is not a guarantee for future lots/products. When applicable, P1 Technologies asks that this requirement be placed on every Purchase Order, within applicable agreements, and/or on drawings/specifications.